Updated February 5th, 2022 to include new Pediatric COVID-19 Vaccine codes and information in advance of FDA EUA approval.
The COVID pandemic has had tremendous emergent impact upon medical care, testing, and supplies. In response, the AMA’s CPT Editorial Panel has had to rapidly create and establish new CPT codes for COVID reporting, tracking and analysis to support data-driven planning and resource allocation.
On November 10, 2020 the AMA announced the coding methodology to be applied for new vaccine and vaccine administration codes that apply specifically to COVID vaccine products. The codes become effective upon Emergency Use Authorization (EUA) approval of the vaccines.
As new vaccines continue to become available, instructional parenthetical notes are added throughout the code set directing coders to the applicable COVID-19 vaccine codes. In addition to the new unique vaccine-specific codes, the AMA releases administration codes unique to each vaccine and dose.
The first COVID Vaccine, a Pfizer-BioNTech product, was granted an Emergency Use Authorization (EUA) on December 12, 2020 allowing providers to begin administering the vaccine to the American public for ages 16 and older. On August 23, 2021, the Pfizer vaccine was granted full FDA approved for the prevention of COVID-19 disease in persons 16 years of age and older. The newer tris-sucrose formulation of the Pfizer vaccine has new codes to distinguish it from the earlier formulation. Previously known as the Pfizer-BioNTech COVID-19 Vaccine, it will now be marketed as Comirnaty. The vaccine continues to be available under the EUA expanded on May 10, 2021 to include those individuals 12 through 15 years of age. In October 2021, the Pfizer EUA was expanded once again to include children ages 5 through 11 years of age.
Moderna’s EUA, for individuals ages 18 and older, was granted December 18, 2020 bringing a second vaccine to the public. Janssen’s single dose vaccine received its EUA on February 27, 2021, but after initial vaccination of the population began, complications were identified and its use was placed on hold before being released again in April 2023 with information about this rare blot clot potential complication included in the amended EUA. The AstraZeneca and Novavax vaccines are continuing through the EUA process; however, the AMA has already provided the codes for these vaccines to give providers the necessary lead time to set them up in their record and billing systems.
The new vaccine administration codes are specific to each coronavirus vaccine as well as to the dose in the required schedule. This level of specificity offers the ability to track each vaccine dose and include the actual work of administering the vaccine, in addition to all necessary counseling provided to patients or caregivers and updating the electronic record. Because the federal government is purchasing and distributing initial vaccines to heath care entities across the country, the administration codes will be especially important in specifying which vaccine has been given as well as dosage and which dose (first or second) was given. Unlike the other vaccines, Janssen’s EUA application is for a single-dose vaccine.
PEDIATRIC COVID-19 VACCINES
On October 29, 2021, the FDA announced EUA of the Pfizer-BioNTech COVID-19 vaccine for children ages 5-11 years. The vaccine is to be administered in a two-dose regimen of 10 mcg/0.2 ml given 21 days apart. This is the first COVID-19 vaccine authorized, in the United States, for children in this age range. The AMA added the codes to Appendix Q of the CPT manual. The codes are listed in the AMA’s table below.
A few days after this approval, CMS announced that coverage for this age group is available without cost-sharing for Medicare, Medicaid, and CHIP. Additionally, most commercial market beneficiaries cover the vaccine and its administration without cost-share.
On February 1, 2022, the AMA issued new CPT® codes for the Pfizer-BioNTech COVID-19 vaccine for children 6 months to under 5 years of age. The issuance of these codes comes in advance of the pending FDA EUA approval. Please see the coding table later in this blog for details.
THIRD DOSE
The FDA had earlier amended the EUAs, August 2021, for both Pfizer and Moderna COVID-19 vaccines to allow for a third dose for qualified immunocompromised people or those with underlying medical conditions, ages 18 and older. CMS announced reimbursement, effective August 12, 2021, for that third dose.
On January 3, 2022, the FDA approved a third dose as part of the primary series for certain immunocompromised children ages 5 -11 as part of the amendment to the Pfizer EUA only. This amendment now makes it possible for certain immunocompromised individuals ages 5 and older to receive a third Pfizer vaccine dose as part of the primary series.
A Moderna third dose for the 5-17 age group has not yet reviewed or approved.
BOOSTER DOSE
Pfizer-BioNTech
The FDA amended the EUA for the Pfizer-BioNTech (September 22, 2021) COVID-19 Vaccine to allow for the use of a single booster dose, to be administered at least 6 months after completion of the primary series (2 doses) for individuals who meet certain criteria. On January 3, 2022, the time from completion of the initial primary series and the booster was lowered to 5 months. The CDC established this guidance to individuals regarding the Pfizer booster shot:
- 12 - 17 years of age receiving only the Pfizer-BioNTech or Comirnaty booster dose. Not a mix-and-match authorization.
- 18 years of age and older (November 19th, 2021 changed from 65 and older to 18 years and older)
- 18 years of age and older who live in long-term care settings or high-risk settings
- 18 years of age and older whose “frequent institutional or occupational exposure to SARS-CoV-2 puts them at high risk of serious complications of COVID-19 including severe COVID-19.”
The codes for the Pfizer booster shots are the same whether the shot is of the original or tris-sucrose formulation. Please see the AMA table below.
Moderna
The FDA amended the EUA for the Moderna (October 20, 2021) COVID-19 Vaccine to allow for the use of a single booster dose, to be administered at least 6 months after completion of the primary series (2 doses) for individuals who meet certain criteria. The CDC established this guidance to individuals regarding the Moderna booster shot:
- 18 years of age and older (November 19th, 2021 changed from 65 and older to 18 years and older)
- 18 years of age and older who live in long-term care settings or high-risk settings
- 18 years of age and older whose “frequent institutional or occupational exposure to SARS-CoV-2 puts them at high risk of serious complications of COVID-19 including severe COVID-19.”
- Note that the Moderna booster is half of a single dose amount.
See the CDC website for more information.
Janssen (Johnson and Johnson)
The FDA amended the EUA for the Janssen (October 20, 2021) COVID-19 Vaccine to allow a booster shot to be administered at least 2 months after completion of the single-dose primary inoculation to individuals:
- 18 years of age and older
Mix and Match
On October 20, 2021, the FDA approved the use of each available COVID-19 vaccine (Pfizer, Moderna, Janssen) as a heterologous booster does in eligible individuals. An eligible individual is one who meets the criteria for each of the previously defined scenarios for each of the vaccines, with the exception of 5-17 year olds.
CMS followed each FDA announcement of the approved EUA-amended use of the COVID-19 vaccines by stating that it will pay for the booster shots.
Third dose and booster shot CPT codes were published by the AMA in advance of these approvals to allow providers time to update their electronic systems. The AMA worked closely with the CDC and FDA to develop the codes. CMS will pay the same amount they pay for earlier doses, approximately $40 each, to administer this additional dose.
AMA President Susan R. Bailey, MD on why these new codes are important: “An effective national immunization program is key to bringing the coronavirus pandemic to an end. Correlating each coronavirus vaccine with its own unique CPT code provides analytical advantages to help track, allocate and optimize resources as an immunization program ramps up in the United States.”
NEW VACCINE AND VACCINE ADMINISTRATION CODES:
A new Appendix Q was also added to the CPT code set and will list the product, administration codes, dosing timelines and manufacturer. This crosswalk will also be used for tracking and reporting outcomes and efficacy of specific vaccines. New codes since the last version of this blog are identified with a

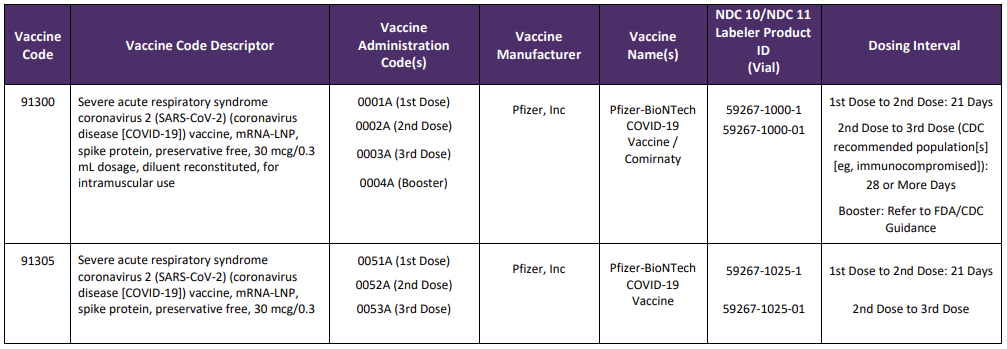
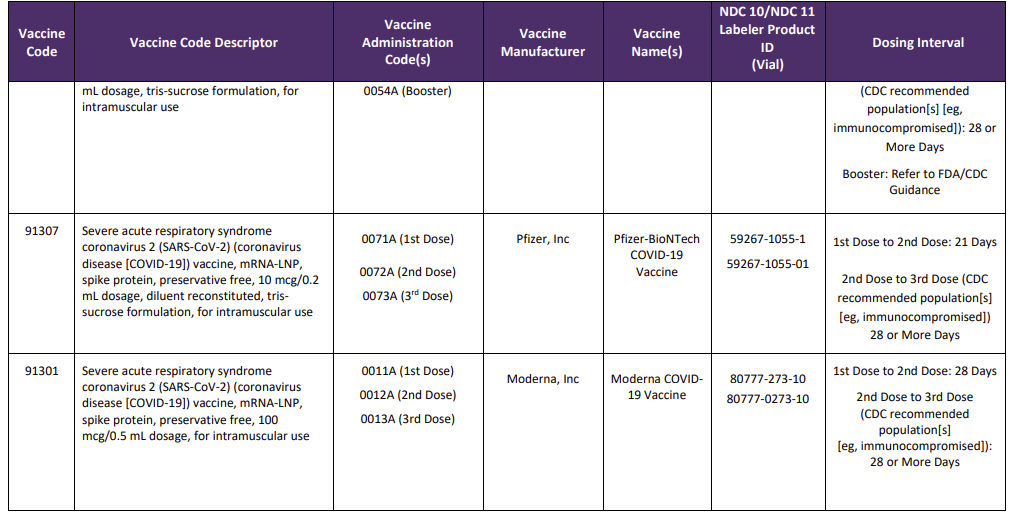
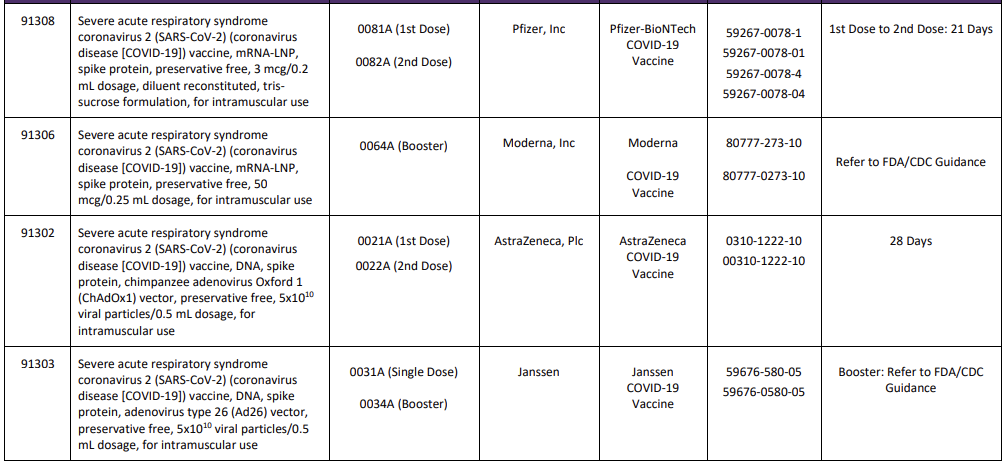

Table Source: https://www.ama-assn.org/sysem/files/2020-11/covid-19-immunizations-appendix-q-table.pdf
It is important to note that 90460—90474 should not be reported for the administration of the SARS-CoV-2 vaccines.
The AMA plans to introduce more vaccine-specific CPT codes as more vaccine candidates are close to being approved for Emergency Use by the FDA. This blog will be updated as more information becomes available.
FAQ’s
Please note that the guidance is changing on an almost-daily basis. It is important to check with your payers to get the most accurate information specific to your health center.
Q: How will Medicare payments to health centers for COVID-19 vaccines and their administration be implemented?
A: Payment will be implemented the same way flu/pneumococcal vaccines are currently provided. According to the NACHC, these vaccines and their administration are paid at 100 percent of reasonable cost through the cost report. No visit is billed if it is a vaccination-only encounter, and these costs should not be included on the claim. Cost sharing is waived. CMS has provided COVID vaccine policies and guidance: https://www.cms.gov/covidvax
Beneficiaries with Medicare pay nothing for COVID-19 vaccines from the federal government, and there is no applicable copayment, coinsurance, or deductible.
Q: What about Medicare Advantage?
A: Prior to January 1, 2022, if you participate in a Medicare Advantage Plan, CMS advises providers to submit the COVID-19 vaccination claims to Original Medicare through your MAC. Medicare Advantage administrative fees are reported on health center cost reports. Medicare Advantage beneficiaries do not pay a cost-share for the vaccine.
As of January 1, 2022, COVID-19 vaccination administration claims for Medicare Advantage are to be submitted to the Medicare Advantage Plan.
Q: How will Medicaid reimburse providers for COVID vaccines and administration?
A: The toxoid vaccine is provided for free to all Medicaid beneficiaries. State Medicaid and CHP agencies must provide vaccine administration without cost sharing to all beneficiaries through to the end of the PHE. Through the American Rescue Act signed by President Biden of March 11, 2021, vaccine administration will be federally funded. As more information is not yet available, It is important to note that Medicaid reimbursement varies by state and type of arrangement (i.e., Managed care or FFS) and communication with the plan’s point-of-contact for details on how to submit claims is important.
Q: We received the COVID vaccine toxoid for free, can we bill for the vaccine administration?
A: The vaccine administration code applicable to the product and dose should be submitted on the claim. For tracking purpose, the applicable vaccine toxoid code should also be submitted with no fee attached.
Q: Some of our patients are private plan beneficiaries. How do we bill for the vaccine and its administration?
A: Most private health plans are required to cover the vaccine and its administration, both in-network and out-of-network, with no cost sharing during the PHE. Medicare reimbursement rates should be referred to as a potential guideline for insurance companies paying a FFS rate. CMS expects commercial carriers to ensure that their rates are reasonable compared with market rates. On March 15, 2021, CMS increased reimbursement to $40 to administer each does of the vaccine.
Q: How will I get reimbursed for administering the COVID vaccine to my uninsured patients?
A: Providers administering the vaccine to people without health insurance or whose insurance does not provide coverage of the vaccine, can request reimbursement for COVID-19 vaccine administration through the CARES Act Provider Relief Fund, see https://www.hrsa.gov/CovidUninsuredClaim. The program will provide vaccine administration codes to use for claims submission on their webpage, so be sure to check there frequently.
Q: If a patient is coming in for a COVID vaccination only, do I use an E&M Code?
A: No, for vaccination-only visits, code the specific vaccine and the corresponding administration code for the vaccine. If the vaccine is free, code only the administration code.
REFERENCE SOURCES:
https://public-inspection.federalregister.gov/2020-26815.pdf
https://www.cms.gov/Center/Provider-Type/Federally-Qualified-Health-Centers-FQHC-Center
https://www.ama-assn.org/practice-management/cpt/covid-19-cpt-coding-and-guidance
https://www.ama-assn.org/system/files/2020-11/cpt-assistant-guide-covid-vaccine-coding-2020.pdf
